Prevalența PID-BTC
Fibroza pulmonară este o amenințare critică într-o gamă largă de pneumopatii interstițiale difuze asociate cu boala țesutului conjunctiv (PID-BTC)1–5

PNEUMOPATIA INTERSTIȚIALĂ DIFUZĂ (PID) ESTE O MANIFESTARE TIMPURIE COMUNĂ A BTC ȘI ARE CĂI PATOGENICE COMUNE CĂTRE FIBROZA1–7
PID alcătuiește un grup divers de peste 200 de afecțiuni pulmonare heterogene, în mare parte clasificate ca fiind rare sau observate doar rar în practica clinică8–10
În timp ce unele PID sunt idiopatice, altele se manifestă ca rezultat al expunerii la antigenele de mediu sau ca o complicație pulmonară a unui BTC subiacent11
În PID-BTC, fibroza pulmonară este caracterizată prin cicatrizarea adesea cronică și ireversibilă a țesutului pulmonar,12,13 și este un factor cheie al leziunilor pulmonare ireversibile și al mortalității precoce în BTC.1–5,14
Care este prevalența PID în BTC?
Estimările de prevalență pentru diferite BTC și PID-BTC variază între diferite studii. Cifrele de prevalență prezentate mai jos se bazează pe o serie de estimări diferite din diverse studii.15–40
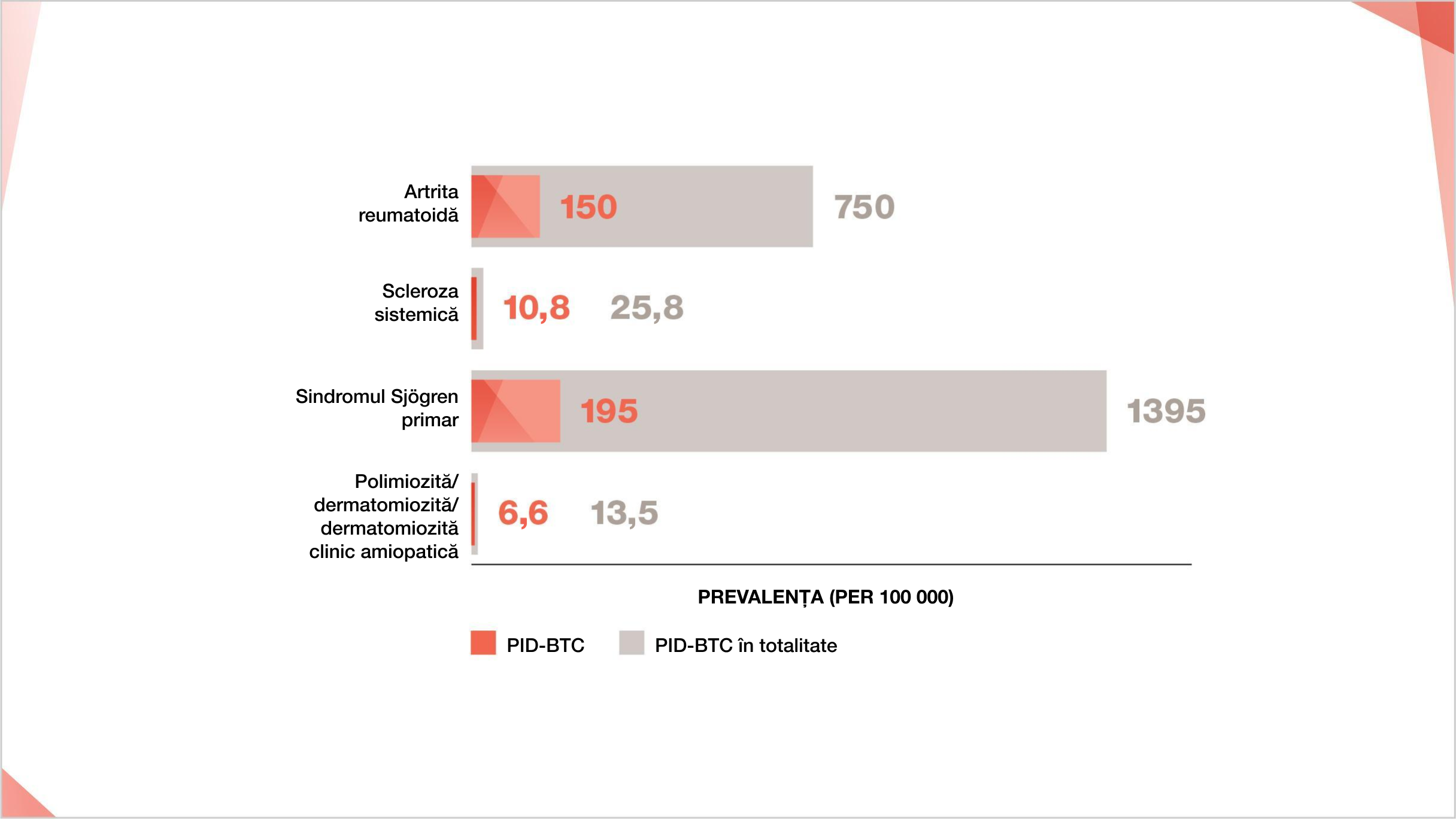
Prevalența BTC și PID-BTC la 100.000
Cifrele de prevalență sunt obținute ca valori medii din intervalele pentru prevalența BTC și PID-BTC, după cum urmează: Prevalența AR 500-1000 la 100 000;15–17 Prevalența PID-AR 10%-30% din AR.18-22 Prevalența ScS 7,2-44,3 la 100 00023 Prevalența PID-ScS 42% din ScS.24 Prevalența sindromului Sjögren primar 90-2700 la 100 00025,26 Sindromul Sjögren primar-Prevalența PID 8%-20% din sindromul Sjögren primar27-30 Prevalența PM/DM 5-22 la 100 00031,32 Prevalența PM/DM/CADM-ILD 20%-78% din PM/DM/CADM.33-40
PID-BTC sunt un subgrup important de PID10
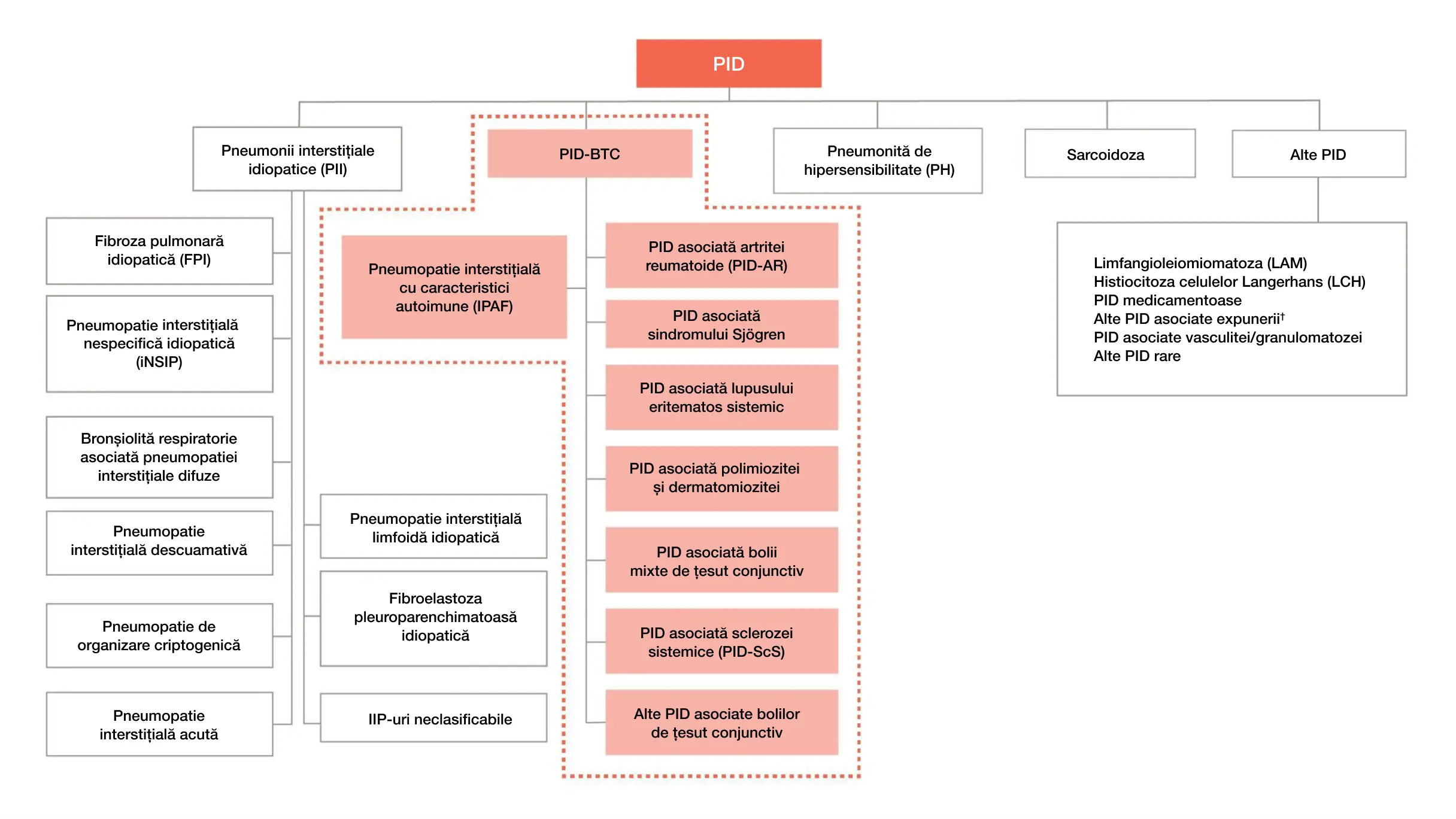
* Nu este un diagnostic clinic estimat
† De exemplu: azbestoza, silicoza
Adaptat după: Cottin V, et al. Eur Respir Rev. 2018;27:180076.


PID se dezvoltă la până la 30%
dintre pacienții cu AR18-22
Aflați mai multe despre prevalența PID-AR

PID se dezvoltă la 8%-20% dintre pacienții cu sindromul primar Sjögren27-30
Aflați mai multe despre prevalența PID asociată sindromului primar Sjögren
DE CE ESTE IMPORTANT DIAGNOSTICUL PRECOCE AL PID?
PID se dezvoltă adesea devreme în cursul evoluției BTC și poate fi chiar prima manifestare a unei BTC nediagnosticată sau nerecunoscută anterior,1,2,5,6 PID în BTC poate fi de natură subclinică (fără simptome),7 poate fi cronic progresivă, sau chiar prezentă într-o manieră care pune viața în pericol.42
Exacerbarea acută a PID, caracterizată prin deteriorarea rapidă a respirației cu hipoxemie severă, poate apărea la pacienții cu PID-BTC în orice moment pe parcursul evoluției bolii.43–47 Pe baza pacienților cu FPI, exacerbarea acută a PID este cel mai probabil declanșată de un eveniment acut, cum ar fi infecția.48 Se raportează că mortalitatea spitalicească post-exacerbare la pacienții cu PID-BTC variază între 50%-100%.43
Ce ar putea însemna PID fibrozantă cronică pentru pacienții dumneavoastră cu BTC?
Creșterea ratei mortalității
Exacerbarea acută a PID în PID-BTC

Cazuri de pacienți PID-BTC
Note
BTC, boala țesutului conjunctiv; PID-BTC, pneumopatie interstițială difuză asociată cu boala țesutului conjunctiv; IIP, pneumonie interstițială idiopatică; PID, pneumopatie interstițială difuză: PH, pneumonită de hipersensibilitate; IPAF, pneumonită interstițială cu caracteristici autoimune; FPI, fibroză pulmonară idiopatică; LAM, limfangioleiomiomatoză; LCH, histiocitoză cu celule Langerhans; NSIP, pneumonie interstițială nespecifică; AR, artrita reumatoidă; PID-AR pneumopatie interstițială difuză asociată cu artrita reumatoidă; ScS, scleroză sistemică; PID-ScS, pneumopatie interstițială difuză asociată sclerozei sistemice.
- Fischer A and Distler J. Progressive fibrosing interstitial lung disease associated with systemic autoimmune diseases. Clin Rheumatol. 2019;38(10):2673–2681.
- Mathai SC and Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ. 2016;352:h6819.
- Wallace B, Vummidi D, Khanna D. Management of connective tissue diseases associated interstitial lung disease: a review of the published literature. Curr Opin Rheumatol. 2016;28(3):236–245.
- Spagnolo P, Cordier JF, Cottin V. Connective tissue diseases, multimorbidity and the ageing lung. Eur Respir J. 2016;47(5):1535–1558.
- Vacchi C, Sebastiani M, Cassone G, et al. Therapeutic options for the treatment of interstitial lung disease related to connective tissue diseases. A narrative review. J Clin Med. 2020;9(2):407. doi:10.3390/jcm9020407.
- Koo SM, Kim SY, Choi SM, et al. Korean guidelines for diagnosis and management of interstitial lung diseases: part 5. Connective tissue disease associated interstitial lung disease. Tuberc Respir Dis (Seoul). 2019;82(4):285–297.
- Doyle TJ, Hunninghake GM, Rosas IO. Subclinical Interstitial Lung Disease. Am J Respir Crit Care Med. 2012;185:1147–1153.
- Flaherty KR, Brown KK, Wells AU, et al. Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of ... in patients with progressive fibrosing interstitial lung disease. BMJ Open Resp Res. 2017;4(1):e000212.
- Demedts M, Wells AU, AntÓ JM, et al. Interstitial lung diseases: an epidemiological overview. Eur Respir J Suppl. 2001;18(suppl32):2s-16s.
- Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150):180076. doi:10.1183/16000617.0076-2018.
- Spagnolo P, Distler O, Ryerson CJ, et al. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs). Ann Rheum Dis. 2021;80:143–150.
- Patterson KC, Strek ME. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann Am Thorac Soc. 2013;10(4):362-370.
- Caban JJ, Yao J, Bagci U, Mollura DJ. Monitoring pulmonary fibrosis by fusing clinical, physiological, and computed tomography features. Conf Proc IEEE Eng Med Biol Soc. 2011;6216-6219.
- Maher TM, Wuyts W. Management of fibrosing interstitial lung diseases. Adv Ther. 2019;36(7):1518–1531.
- Assayag D, Lee JS, King Jr TE. Rheumatoid arthritis associated interstitial lung disease: a review. Medicina (B Aires). 2014;74(2):158–165.
- Kawano-Dourado L, Doyle TJ, Bonfiglioli K, et al. Baseline characteristics and progression of a spectrum of interstitial lung abnormalities and disease in rheumatoid arthritis. Chest. 2020:S0012-3692(20)31412-4. doi:10.1016/j.chest.2020.04.061.
- Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27(2):269–281.
- Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid Arthritis–Interstitial Lung Disease–associated Mortality. Am J Respir Crit Care Med. 2011;183:372–378.
- Geerts S, Wuyts W, de Langhe E, et al. Connective tissue disease associated interstitial pneumonia: a challenge for both rheumatologists and pulmonologists. Sarcoidosis Vasc Dif. 2017;34:326–335.
- Esposito AJ, Chu SG, Madan R, et al. Thoracic manifestations of rheumatoid arthritis. Clin Chest Med. 2019;40(3):545–560.
- Shaw M, Collins BF, Ho LA, Raghu G. Rheumatoid arthritis-associated lung disease. Eur Respir Rev. 2015;24(135):1–16.
- Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics – a large multicentre UK study. Rheumatology (Oxford). 2014;53(9):1676–1682.
- Bergamasco A, Hartmann N, Wallace L, et al. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin Epidemiol. 2019;11:257–273.
- Walker UA, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis. 2007;66(6):754-763. doi:10.1136/ard.2006.062901.
- Alamanos Y, Tsifetaki N, Voulgari PV, et al. Epidemiology of primary Sjögren’s syndrome in north-west Greece, 1982–2003. Rheumatology. 2006;45:187–191.
- Patel R, Shahane A. The epidemiology of Sjögren’s syndrome. Clin Epidemiol. 2014;6:247–255.
- Watanabe M, Naniwa T, Hara M, et al. Pulmonary Manifestations in Sjögren’s Syndrome: Correlation Analysis Between Chest Computed Tomographic Findings and Clinical Subsets with Poor Prognosis in 80 Patients.
J Rheumatol. 2010;37:365-373. - Sambataro G, Ferro F, Orlandi M, et al. Clinical, morphological features and prognostic factors associated with interstitial lung disease in primary Sjögren’s syndrome: A systematic review from the Italian Society of Rheumatology. Autoimmun Rev. 2020;19:102447.
- Ramos-Casals M, P Brito-Zerón, Seror R, et al. Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology. 2015;54:2230-2238.
- Roca F, Dominique S, Schmidt J, et al. Interstitial lung disease in primary Sjögren’s syndrome. 2016;http://dx.doi.org/10.1016/j.autrev.2016.09.017.
- Cheeti A, Brent LH, Panginikkod S. Autoimmune Myopathies. [Updated 2020 Aug 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532860/. Last accessed Jan 2021.
- Yang SH, Chang C, Lian Z-X, et al. Polymyositis and dermatomyositis – challenges in diagnosis and management. J Transl Autoimmun. 2019;2:100018.
- Li R, Zhu W-J, Wang F, et al. AST/ALT ratio as a predictor of mortality and exacerbations of PM/DM-ILD in 1 year-a retrospective cohort study with 522 cases. Arthritis Res Ther. 2020;22(1):202.
- Shappley C, Paik JJ, Saketkoo LA. Myositis-related interstitial lung diseases: diagnostic features, treatment, and complications. Curr Treatm Opt Rheumatol. 2019;5(1):56–83.
- Fathi M, Vikgren J, Boijsen M, et al. Interstitial lung disease in polymyositis and dermatomyositis: longitudinal evaluation by pulmonary function and radiology. Arthritis Rheum. 2008;59(5):677–685.
- Yu K, Wu YJ, Kyo C, et al. Survival analysis of patients with dermatomyositis and polymyositis: Analysis of 192 Chinese cases. Clin Rheumatol. 2011;30:1595-1601.
- Ji S-Y, Zeng F-Q, Guo Q, et al. Predictive factors and unfavourable prognostic factors of interstitial lung disease in patients with polymyositis or dermatomyositis: a retrospective study. Chin Med J (Engl). 2010;123(5):517–522.
- Chen I-J, Jan Wu Y-J, Lin C-W, et al. Interstitial lung disease in polymyositis and dermatomyositis. Clin Rheumatol. 2009;28(6):639–646.
- Hayashi S, Tanaka M, Kobayashi H, et al. High-resolution computed tomography characterization of interstitial lung diseases in polymyositis/dermatomyositis. J Rheumatol. 2008;35(2):260–269.
- Kang EH, Lee EB, Shin KC, et al. Interstitial lung disease in patients with polymyositis, dermatomyositis and amyopathic dermatomyositis. Rheumatology (Oxford). 2005;44(10):1282–1286.
- Hoffmann-Vold AM, Fretheim H, Halse AK, et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med. 2019;200;1258–1266.
- Song JW, Lee H, Lee C, et al. Clinical Course and outcome of rheumatoid arthritis-related usual interstitial pneumonia. Sarcoidosis Vasc Dif. 2013;30:103–112.
- Kolb M, Bondue B, Pesci A, et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150):pii:180071.
- Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Resp Med. 2009;103:846–853.
- Cao M, Sheng J, Qiu X, et al. Acute exacerbations of fibrosing interstitial lung disease associated with connective tissue diseases: a population-based study. BMC Pulm Med. 2019;19:215.
- Tomiyama F, Watanabe R, Ishii T, et al. High Prevalence of Acute Exacerbation of Interstitial Lung Disease in Japanese Patients with Systemic Sclerosis. Tohoku J Exp Med. 2016;239, 297–305.
- Okamoto M, Fujimoto K, Sadohara J, et al. A retrospective cohort study of outcome in systemic sclerosis-associated interstitial lung disease. Respiratory Investigation. 2016;54, 445–453.
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis – An International Working Group Report. Am J Respir Crit Care Med. 2016;194:265–275.
- Song JW, Hong S-B, Lim C-M, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–363.
